The meiofauna as neglected carriers of antibiotic resistant and pathogenic bacteria in freshwater ecosystems
Accepted: 22 November 2021
HTML: 92
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
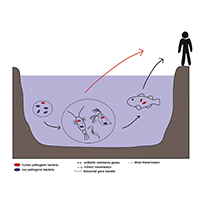
The World Health Organization considers antibiotic resistance as one of the main threats to human and other animals' health. Despite the measures used to limit the spread of antibiotic resistance, the efforts made are not enough to tackle this problem. Thus, it has become important to understand how bacteria acquire and transmit antibiotic resistant genes (ARGs), in particular in the environment, given the close connection between the latter and human and animal health, as defined by the One-Health concept. Aquatic ecosystems are often strongly impacted by anthropogenic activities, making them a source for ARGs and antibiotic resistant bacteria (ARB). Although freshwater meiofauna have been the object of active research, few studies have focused on the relationship between the spread of antibiotic resistance and these organisms. In this review, we investigated freshwater meiofauna as carriers of resistances since they play a central role in the aquatic environments and can harbor human and animal potential pathogens. We assessed if these animals could contribute to the spread of ARGs and of potentially pathogenic bacteria. Only four taxa (Rotifera, Chironomidae, Cladocera, Copepoda) were found to be the subject of studies focused on antibiotic resistance. The studies we analyzed, although with some limitations, demonstrated that ARGs and ARB can be found in these animals, and several of them showed the presence of potentially pathogenic bacteria for humans and animals within their microbiome. Thus, meiofauna can be considered a source and a reservoir, even if neglected, of ARGs and ARB for the freshwater environments. However, further studies are needed to evaluate the impact of the meiofauna on the spread and persistence of antibiotic resistance in these ecosystems.
Supporting Agencies
Cariplo Foundation (WARFARE project, grant n° 2018-0995), International Commission for the Protection of Italian-Swiss Waters (“INDAGINI LIMNOLOGICHE SUL LAGO MAGGIORE” program)How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Similar Articles
- Juliana V. Rangel, Rosângela E.S. Araújo, Cinthia G. Casotti, Larissa C. Costa, Walace P. Kiffer Jr., Marcelo S. Moretti, Assessing the role of canopy cover on the colonization of phytotelmata by aquatic invertebrates: an experiment with the tank-bromeliad Aechmea lingulata , Journal of Limnology: Vol. 76 No. 2 (2017)
- Jong-Yun Choi, Kwang-Seuk Jeong, Geung-Hwan La, Seong-Ki Kim, Gea-Jae Joo, Sustainment of epiphytic microinvertebrate assemblage in relation with different aquatic plant microhabitats in freshwater wetlands (South Korea) , Journal of Limnology: Vol. 73 No. 1 (2014)
- Andrea Di Cesare, Ester Eckert, Gianluca Corno, Co-selection of antibiotic and heavy metal resistance in freshwater bacteria , Journal of Limnology: Vol. 75 No. s2 (2016): Lake Orta: a new lease on life
- Maximiliano D. Garcia, Nicolás Bonel, Environmental modulation of the plankton community composition and size-structure along the eutrophic intertidal coast of the Río de la Plata estuary, Argentina , Journal of Limnology: Vol. 73 No. 3 (2014)
- Zacarias Fresno Lopez, Tommaso Cancellario, Diego Fontaneto, Lyudmila Kamburska, Karimullah Karimullah, Robert L. Wallace, Elizabeth J. Walsh, Radoslav Smolak, A georeferenced dataset for occurrence records of the phylum Rotifera in Africa , Journal of Limnology: Vol. 82 No. s1 (2023): Georeferenced freshwater biodiversity data
- Csaba Vadadi-Fülöp, Levente Hufnagel, Climate change and plankton phenology in freshwater: current trends and future commitments , Journal of Limnology: Vol. 73 No. 1 (2014)
- Cristian Granados-Martínez, Bladimir Zúñiga-Céspedes, Julio Acuña-Vargas, Diets and trophic guilds of aquatic insects in Molino River, La Guajira, Colombia , Journal of Limnology: Vol. 75 No. s1 (2016): Proceedings of the 6th National Congress of Limnology
- Mircea Alexe, Gheorghe Șerban, Andreea Baricz, Adrian-Ștefan Andrei, Adorján Cristea, Karina P. Battes, Mirela Cîmpean, Laura Momeu, Vasile Muntean, Sebastian A. Porav, Horia L. Banciu, Limnology and plankton diversity of salt lakes from Transylvanian Basin (Romania): A review , Journal of Limnology: Vol. 77 No. 1 (2018)
- Michał Niedźwiecki, Malgorzata Adamczuk, Tomasz Mieczan, Trophic interactions among the heterotrophic components of plankton in man-made peat pools , Journal of Limnology: Vol. 76 No. 3 (2017)
- Xu Sun, Aili Wang, Liuyan Yang, Liyun Guo, Qiankun Chen, Zhinxin Hu, Lijuan Jiang, Lin Xiao, Spatial distribution of ammonia-oxidizing archaea and bacteria across eight freshwater lakes in sediments from Jiangsu of China , Journal of Limnology: Vol. 73 No. 2 (2014)
You may also start an advanced similarity search for this article.
-
Shijie Jia, Xiaohong Yao, Jianhua Qi, Xiaohuan Liu, Huiwang GaoFrontiers in Marine Science : 2025
-
Francesca Falco, Aurora Dibra, Ledia Vasjari, Fundime Miri, Maria Pagano, Eliana Ibrahimi, Valbona AlikoThe Handbook of Environmental Chemistry : 2024
-
Andrea Di Cesare, Raffaella Sabatino, Tomasa Sbaffi, Diego Fontaneto, Diego Brambilla, Andrea Beghi, Franca Pandolfi, Cristina Borlandelli, Davide Fortino, Giovanni Biccai, Pietro Genoni, Gianluca CornoChemosphere : 2023

 https://doi.org/10.4081/jlimnol.2021.2054
https://doi.org/10.4081/jlimnol.2021.2054





